Directions, the following were done chronologically:
-
- We measured 20 milliliters of the following, each in different cups:
- Lemon juice
- Vinegar
- Sprite
- Water
- A water-and-toothpaste solution
- A water-and-baking-soda solution
- Bleach
- A small amount of red cabbage indicator was added into each of the cups
- The lemon juice was poured into the bleach
- The baking soda solution was mixed with the vinegar
- The sprite was poured into the toothpaste solution
- We measured 20 milliliters of the following, each in different cups:
Report/Response:
*The following image was used as the pH scale for the indicator
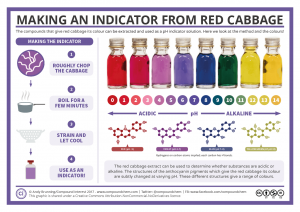
Using a dropper, my teammate squished and a few drops of red cabbage juice fell into the first cup which contained lemon juice. The color change that took place was what me and my teammates expected. The lemon juice went from yellow to pinkish red – the color of a peach – meaning that it was acidic. We used the pH scale above to identify the acidity of the lemon juice and everyone agreed that the level of acidity was somewhere between 1 and 2, so it was really acidic. This process was repeated for the remaining cups, below are the color changes and pH levels:
- The vinegar turned pink and had a pH level of 2; acidic.
- The sprite turned light purple and had a pH level of 3; acidic.
- The water didn’t really change color, it turned into a very light purple. It had a pH level somewhere around 7; it was balanced.
- The toothpaste-and-water solution changed from a very light teal into a darker type of teal, so we agreed that it had a pH level of 11; it definitely had base.
- The baking-soda-and-water solution turned teal similar to the toothpaste-and-water solution, but it slightly lighter. It had a pH of 10; it had base, too.
- The last one was the cup of bleach. It didn’t change much. It was still transparent, but in terms of color, it got more yellow; it turned into a very light yellow. My team met and discussed. We agreed that the bleach probably had a pH level that was between 11 and 12; it had a lot of base.
After finally identifying the pH levels of all the seven cups, we took the indicated cup of lemon juice and poured it into the cup of indicated bleach. The result was surprising to me, the color of the solution was similar to the bleach’s color, but slightly darker and it became warm. My team didn’t expect it, in any case of acids and bases, to become warm, but it did. The indicated baking-soda-water-and solution was then poured into the cup of indicated vinegar. Again, the reaction was surprising, the solution turned from pink and teal into purple and it exploded a tiny bit. The tiny explosion, or rather burst, was what was surprising. Three cups were left. We took the indicated cup of sprite and poured it into the toothpaste-and-water solution. Because the past two mixes surprised, we expected there to also be something new, but there didn’t seem to be any sort of noticeable and weird reaction. The solution just turned from light purple and teal to pink.
