Introduction
Dragon Ball is undoubtedly one of the most well-known Japanese manga and anime series in the world. If you have seen it, you would know that it is all about incredible strength and epic battles that decide the fate of the universe. You would also be familiar with beings known as Saiyans, because they play quite a substantial role in the show. You know, those warriors who can change hair colors and transform into giant rampaging ape-like creatures. Shown throughout the series, Saiyans are insanely strong. You have scenes of fighters knocking each other into mountains, piercing bodies with nothing but their bare hands, repeatedly firing powerful blasts of energy, and even destroying entire planets. Just take a look at this 2-minute clip from the fourth episode of Dragon Ball Z to get a glimpse of their strength. How strong are Saiyans?
In this mini report, we will briefly explore and endeavor to answer the following questions:
- How innately strong are Saiyans? How powerful are they compared to humans?
- Is it possible to be that strong in the real world?
For those who have not seen Dragon Ball, Saiyans are a species of superhuman extraterrestrial beings who are generally violent, almost always fighting and seeking for more physical power. The protagonist of the show, Goku, is a Saiyan.
Analysis
So what makes Saiyans so strong? One of the major reasons Saiyans are as physically capable as they are is their evolution. The planet they lived on, Planet Sadala, was a celestial body whose gravity was 10 times greater than Earth’s. What does that mean? Well, first, it means that if a human (weighing 62 kg on average) were to be on Planet Sadala, it would be as if a European bison, weighing about 610 kg, were on top of you.
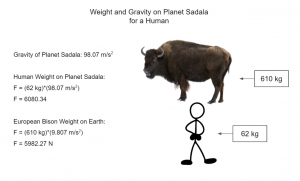
According to research, the maximum gravity that the average human body could withstand is 5 times the Earth’s gravity, or 49.03500 m/s2. Multiply that value by 2 and you get the gravity of Planet Sadala, 98.07 m/s2. Humans wouldn’t be able to survive, or even stand, on the planet due to the significant increase in weight. This illustrates how much “harsher” the Saiyan environment is compared to our human environment.
Secondly, this means that Saiyans can move around on Earth fairly easily. Since Earth’s gravity is 10 times less than that of their home planet, they would be 10 times more agile. For example, if a Saiyan can jump 0.5 m on Planet Sadala, they would be able to jump 5 m on Earth. Oppositely, if a human can jump 0.5 m on Earth, they would be able to “jump” (or try to jump) only 5 cm on Planet Sadala. Based on the difference between our planet’s gravity and that of the home planet of Saiyans, we can gather that Saiyans are basically about 10 times stronger than us humans.
Let’s take a look at a scene from the anime to better our understanding.

In one of the beginning episodes of Dragon Ball, after Goku, of age 12, is hit by a car, he goes to lift it up and throw it because he thinks it’s a monster. That car was the 1980-1984 Renault 5 Turbo, which has an unladen mass of 980 kg! Additionally, the car had the female character Bulma in it. According to the Dragon Ball Wiki, Bulma, a teenager at the time, was 48 kg. Now let’s do some math.
F = (980+48 kg)*(9.807 m/s2) F = 10081.596 N
We found the vehicle’s total weight, but we don’t know Goku’s. And I wasn’t able to find his mass either, so we’re going to have to make some assumptions. The average weight (mass) of a 12-year-old human boy is between 67 and 130 pounds, or 30 and 59 kg. We can take an average from the two ends of the range, and get 45 kg. We’ll assume that was little-boy Goku’s mass.
F = (45 kg)*(9.807 m/s2) F = 441.31500 N
How many times Goku’s weight was the Renault 5 Turbo with Bulma in it?
(10081.596 N)/(441.31500 N) = 22.8444444 ≈ 23
At only 12 years old, Goku was already capable of lifting almost 23 times his own weight. How crazy is that? For comparison, let’s take a look at the greatest known amount of weight that has ever been lifted by a human in the real world. It is quite a feat.

In the Guinness Book of World Records, on June 12, 1957, 25-year-old Paul Anderson, who weighed 364 lbs or around 165 kg, lifted the greatest weight ever recorded: a whopping 6270 lbs, or roughly 2844 kg! Now how does he compare to 12-year-old Goku from Dragon Ball? Let’s find the weight of his… weight and the weight of his body.
1. Weight of Weight (if that makes any sense at all) 6270 lbs ≈ 2844 kg F = (2844 kg)*(9.807 m/s2) F = 27891.108 N 2. Weight of Body 364 lbs ≈ 165 kg F = (165 kg)*(9.807 m/s2) F = 1618.155 N 3. (27891.108)/(1618.155 N) = 17.2363636 ≈ 17
Here, we see that Paul Anderson was able to lift approximately 17 times his own weight at 25 years old, while Goku was able to lift 23 times his own weight at only 12 years old. That is just bonkers. This proves that Saiyans truly are beings with incredible physical strength. If Goku was that strong as a kid, imagine how strong he is as an adult! *If you are caught up on the show, you don’t have to imagine.
But in Dragon Ball, a lot more tends to be at play. In addition to natural characteristics, many more things are factors of physical strength, not just for Saiyans but, essentially, all living beings, including humans. This is where we start to involve Power Level and Ki. They’re two of the most fundamental concepts related to strength in Dragon Ball. If you have not heard of these two terms, here is a brief explanation:

Ki is basically the “life force energy” of any living thing. It is supposedly tangible and normally found at the center of an organism’s body. Fighters can bring out Ki and manipulate it in many different ways. It can be turned into blasts of energy or utilized to transform users and make them more powerful, like how Goku changes his hair color in the picture above. Ki and physical form are dependent on each other. The stronger an individual’s body is, the more Ki they have, or vice versa. What this means is that you can get stronger beyond your natural abilities by training further and gaining more Ki.
As for power level, it is essentially a rough assessment of one’s physical ability based on the amount of Ki they possess. The instrument most commonly used to determine the power levels of characters in the series is the scouter. Here is what it looks like:
Technically, power levels are the numbers (of an unstated unit) given by this gadget when used. They give fighters a sense of who is stronger and how battles will likely end. For example, if someone has a power level of 40, then they would have a good chance of winning against someone with a power level of 13.
The physical nature (in both senses of the word “physical”) of Ki isn’t discussed or explained in detail in Dragon Ball, but we can get an idea of how strong characters are compared to each other in terms of physical strength by looking at their power levels, since power levels are approximations of Ki amount and Ki and physical strength determine (are directly proportional to) each other.
On the Dragon Ball Wiki, it is said that the power level of the average human being is between 5 and 10. Let’s say 8 (I took an average of the two numbers and rounded to the nearest whole number). As for Saiyans… Well, it isn’t specified, but Goku’s brother Raditz, who was a mid-class warrior, had a power level of 1,500, so we’ll use that value as our average Saiyan power level. What this means is that in addition to their natural strength, Saiyans are also about 188 times (1500/8 = 187.5) more powerful than humans because they generally have so much more Ki, and therefore, physical strength.
The average human (untrained, that is) can punch with a pressure that is anywhere between 60 and 85 psi. We’ll use 73 psi (again, I took the average and rounded). Let’s convert that number into kPa (kilopascals) since it is going to make it easier to work with, and we get around 503 kPa. Now, if we disregard power levels and Ki, Saiyans would be able to punch 10 times harder than humans (since they are innately 10 times stronger than us due to their evolution), with a pressure of 5030 kPa. But if we factor power levels and Ki into the equation, they would be able to punch about 188 times harder than us, with a pressure of 94564 kPa, which just sounds absurd. How much force is that? Well, the definition of pressure is “force per unit area,” (P = F/A) so we can use that formula to figure out how much force the average Saiyan punch produces.
But we don’t know the area! That’s fine. The area here is simply the front area of the Saiyan fist because the fist is what’s exerting the force here. Since the appearance of Saiyans do not differ very much from that of humans, I took the average hand size of male adult humans and calculated the area of a fist. You can follow my process here:
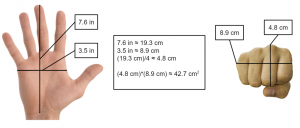
Now, we can convert 42.7 cm2 to 0.427 m2 since the unit kilopascal/pascal calls for meters, and substitute our values into the equation of pressure to find force:
Force of Saiyan Punch Without Ki 5030 kPa = F/(0.427 m2) (5030 kPa)*(0.427 m2) = 2147.81 N ≈ 2148 N Force of Saiyan Punch With Ki 94564 kPa = F/(0.427 m2) (94564 kPa)*(0.427 m2) = 40378.828 N ≈ 40379 N
That is a lot of Newtons. Let’s put those numbers into perspective.
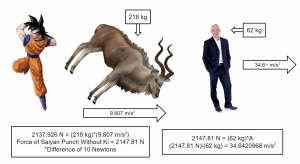
Without considering Ki, if a Saiyan were to punch the average human, the force would be similar to the weight of an adult greater kudu, which weighs around 218 kg. So it would be as if they were hit by a greater kudu accelerating at 9.807 m/s2. That would cause them to accelerate at a rate of about 34.6 meters per second squared.
Or… If this analogy works better for you, it’d be as if they were hit by a 181-kg motorcycle accelerating at 11.8 m/s2 (2135.8 N = (181 kg)*(11.8 m/s2)). Either way, they probably wouldn’t survive. Now, considering Ki…
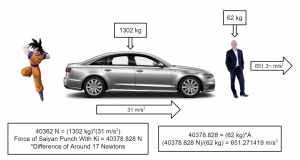
Let’s just say that the average human would have an even slimmer chance of surviving if they were to be hit by the average Saiyan warrior. It would be as if a 1302-kg car were accelerating at them at a rate of 31 meters per second squared, which would cause them to accelerate at about 651.3 m/s2.
These numbers are all hypothetical and may seem ridiculous, but how physically possible is it to be as strong as Saiyans? I believe the closest thing you can get to Saiyan strength in real life is gorilla strength. A gorilla is believed to be able to generate between 1300 and 2700 pounds of force in a single punch, which, apparently, is enough to make your skull shatter. That’s between roughly 5783 and 12010 Newtons! If we take the higher value, 12010 Newtons, and compare it to the punch forces of Saiyans, we see that it is approximately 5.6 times (12010/2148 ≈ 5.6) greater than the natural average Saiyan punch force (without Ki) and that it is about ³⁄₁₀ (12010/40379 ≈ 0.3) of the average Saiyan punch force (with Ki). Well now those values don’t seem super unrealistic anymore—at least, not to me—since a gorilla is already stronger than a Saiyan in terms of innate strength.
But let’s talk a little bit about Ki, because it is what allows the majority of Saiyans to be 18.8 times (40379/2148 ≈ 18.8) stronger than their inherent selves. In the Dragon Ball Universe, it is said that Ki is tangible energy. But we know that in reality, as far as we know, energy is intangible, so whether or not Ki could exist in our world and work the same way it does in Dragon Ball is still a mystery to us; an in-depth examination of Ki would have to be its own paper. For the sake of simplicity in our report, we’ll eliminate Ki on the basis that energy being tangible is unlikely—from our current knowledge of the universe.
Another thing we could consider is physiology since it encompasses the workings of the muscular system, and thus physical strength, but unfortunately, virtually no information is available on Saiyan physiology. However, given that Saiyans and humans seem to be incredibly alike, one could use human physiology as a model to figure out and possibly understand Saiyan physiology. Again, it would have to be its own paper, but for now, it is another obscurity to us.
Conclusion
So how strong are these beings called “Saiyans” from the Japanese animated series Dragon Ball? They are extremely strong. How strong are they when compared to humans? Since the gravity of their original planet is 10 times greater than Earth’s, I would say that they are at least 10 times stronger than humans. Is it physically possible for Saiyans to be as strong as they are, in the show, in the real world? The answer(s) to that question is unclear. From our analysis, we see that a gorilla is actually about 5.6 times stronger than a Saiyan, if we think about natural strength, so one of the answers is: yes, it is physically possible to be 10 times stronger than humans. But if we include Ki on the canvas, that’s a completely different painting. Personally, I believe that it’s somewhat unlikely—to be capable of exerting roughly 40379 Newtons of force in a single punch—but considering that the punch force of a gorilla is ³⁄₁₀ of the average Saiyan punch force with Ki, I also believe that it’s not impossible. I hold the belief that we humans know very little about how all of the universe works, so for now, I’ll leave the question for you, the reader, to decide and answer.
Model/Major Inspiration
https://etnamodfyzx.wordpress.com/2013/04/30/on-the-physics-of-dragon-ball-z/
References
https://aapt.scitation.org/doi/10.1119/1.5124276
https://barbend.com/how-paul-anderson-became-one-of-historys-strongest-humans/
https://dragonball.fandom.com/wiki/Bulma
https://dragonball.fandom.com/wiki/Car
https://dragonball.fandom.com/wiki/Goku
https://dragonball.fandom.com/wiki/Ki
https://dragonball.fandom.com/wiki/Power_Level
https://dragonball.fandom.com/wiki/Raditz
https://dragonball.fandom.com/wiki/Saiyan
https://kpcombat.ca/punching-in-a-street-fight
https://thewebsiteofeverything.com/animals/mammals/adult-weight.html
https://www.autoevolution.com/cars/renault-5-turbo-1980.html
https://www.creditdonkey.com/average-weight-car.html
https://www.healthline.com/health/what-is-the-average-weight-for-a-12-year-old
https://www.livescience.com/36470-human-population-weight.html
https://www.medicalnewstoday.com/articles/average-hand-size
https://www.survivaltechshop.com/motorcycle-weight/
https://www.wildgorillasafaris.com/facts-about-gorilla-facts/how-strong-is-a-gorilla/